Abstract
Background: Acute myeloid leukemia (AML) is the most frequent indication for allogeneic hematopoietic cell transplantation (alloHCT) in the world. According to international recommendations, intermediate and high-risk patients, <75 years and fit should receive consolidation with alloHCT. The role of autologous HCT in AML is less clear. We previously reported in a multicenter retrospective study a very low HCT rate (8.2%) despite most patients being potential candidates. The HCT rates in Latin America are considerably lower than in US or Europe (63.9 vs. 511.2 and 390.0/10 million). The main objective of this study was to analyze the reasons why the patients that were potential HCT candidates were not transplanted.
Methods We included the patients from our previous retrospective multicenter registry that were potential HCT candidates considering: AML diagnosis between January 2013 and December 2017, <66 years, candidates for intensive chemotherapy and with intermediate or high cytogenetic risk.
We reviewed the charts to collect information about HCT decisions and the factors associated with access to transplantation.
Results We included 301 patients from 10 public centers with a median age of 42 years (14-65), 51.8% were women, 93.2% with an ECOG performance status 0-2 and 91% with an HCT-CI <3.
The majority (86.7%) were classified as NOS-AML and 11.6% were secondary AML. Considering cytogenetics: 66.5% had an intermediate risk and 33.5% unfavorable risk. Induction related mortality was: 17.6% and 13.5% were primary refractory.
The majority of the patients (80%) were treated in centers with a local transplant progarm.
HLA testing was performed in 103 patients (34.2%). The main reasons for not performing the tests were: early relapse or primary refractory in 26.3% and lack of access or financial resources in 26.3%. In 7.5% of the cases, the patient refused to be tested. Age >60 years and HCT-CI >3 were associated with a lower likelihood of undergoing HLA matching (OR 0.2 95% CI 0.04-0.69, p=.006 and OR 0.02 95% CI 0.01-0.46, p<.001, respectively), in contrast with the presence of a local transplant program (OR 3.6 95% CI 1.7-7.71, p<.001).
An HLA matched sibling-donor was identified in 45.1%, a haploidentical donor in 32.4% and no donor in 22.5%
Only 23.6% of the patients had a HCT consultation and only 36 patients (12%) received HCT as consolidation in first remission: 30 alloHCT and 6 autoHCT. Only 47.9% of the patients receiving a transplant consultation were transplanted; 20/37 relapsed before HCT. The patients with a matched sibling-donor had a higher probability to receive consolidation with HCT when compared to patients with haploidentical or no sibling donor: 50%, 15.2% and 13% (OR 6 95% CI 2.3-15.5, p<.001). All patients without a matched or half-matched sibling donor who underwent transplantation received autoHCT.
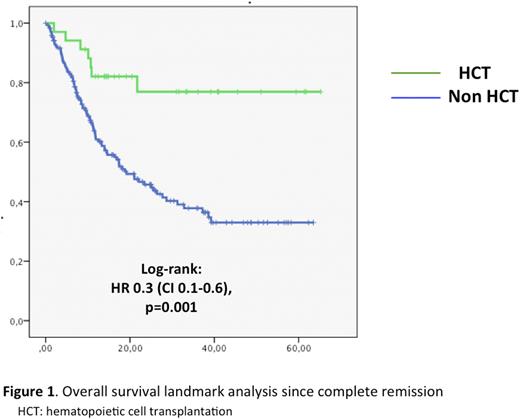
The median time from diagnosis to HCT consolidation was 7.2 months (2.9-25.1 months). A landmark OS analysis since CR showed a significant benefit from HCT (autoHCT or alloHCT): 3-year OS of 76.9% vs. 37.8%; HR 0.3 (0.1-0.6), p=.001. There was no significant difference between autoHCT and alloHCT with a 3-year OS of 83.3% and 75.4%.
Discussion Only 12% of the patients that are potentially candidates are actually receiving HCT in Mexico. HLA testing is performed in only one third of the patients. Lack of access or financial resources and relapses before HLA testing or before HCT are the main reasons. The long-term OS of patients receiving HCT in excellent, however there is a clear bias if with consider the patients that had not the chance to reach the transplant due to early relapses. We have a very big challenge in the region, which is to ensure that the majority of patients who are potential candidates for HCT can access it through timely access to HLA testing and early access to alternative donor HCT.
Disclosures
Delgado:Amgen: Honoraria; Pfizer: Honoraria; Bristol: Honoraria. Montano Figueroa:Abbvie: Consultancy; Janssen: Consultancy. Leyto:Takeda: Consultancy; BMS: Consultancy; Roche: Consultancy; Abbvie: Consultancy; Bayer: Consultancy. Solís-Poblano:Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria. Gomez-De Leon:AbbVie: Honoraria, Other: advisory board. Amador:Abbvie: Consultancy, Honoraria; BMS: Consultancy, Honoraria. García-Castillo:Roche: Consultancy, Honoraria; Asofarma: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Terreros:Roche: Honoraria; Pfizer: Honoraria. Jiménez:Sanofi: Honoraria. Gomez-Almaguer:Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Demichelis:Abbvie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; ASH: Research Funding; Gilead: Consultancy; Teva: Consultancy; Astellas: Consultancy, Honoraria; Amgen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal